COVID-19 antibody tests can usually detect the presence of antibodies in the blood of patients who have been infected with the disease. Most of the tests require only a quick blood stick and can be evaluated on site.
Testing accuracy is uncertain so far; a positive test result may not indicate immunity and a negative result may not show susceptibility.
Currently, antibody tests are being used in seroprevalence surveys being used to track the spread of COVID-19. They may eventually be useful for protecting vulnerable individuals by determining who among the population has developed immunity to the disease and who is still susceptible.
Antibody Testing is One of Several Approaches in COVID-19 Testing
There are basically four different types of tests that can be used to diagnose coronavirus in individuals:
- Reverse Transcription Polymerase Chain Reaction (RT-PCR, but usually just referred to as PCR)
- Loop-Mediated Isothermal Amplification (LAMP)
- Lateral flow
- Enzyme-linked Immunoabsorbent Assay (ELISA)
While LAMP and PCR tests detect the RNA of the virus pathogen itself, lateral flow and ELISA assays detect antibodies that are produced in the body in reaction to the presence of the virus. PCR testing is currently the most common diagnostic test used; almost all figures that are being released by various agencies discussing COVID-19 infections have been established through PCR tests.
Another type of test, a serum neutralization test, can not only find antibodies but also determine their effectiveness by evaluating how they react to actual virus samples in lab-processed cell cultures. Serum neutralization tests are more commonly used in research than disease or antibody diagnostics.
How Does Antibody Testing Work?
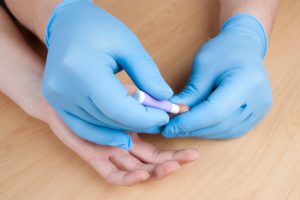
ELISA tests require laboratory processing and are not likely to be extensively used in COVID-19 antibody testing. The lateral flow test is the format that most new tests are being released in.
A lateral flow test requires a small sample of the patient’s blood—a finger-prick will do—which is then dropped on a spongey pad in the test device. A buffer liquid is added to help the blood flow through the sample strip. Chemicals embedded in the strip that are reactive to the antibodies will cause color changes in the strip medium, showing the presence of one or more types of antibodies. A small control strip verifies that the test is operating properly. If for some reason the control strip doesn’t activate, it’s likely the test didn’t perform correctly and should be redone with a new strip.
Two types of antibodies can be detected:
- IgM – These are prototype antibodies, produced early on (within 5 days) of a new infection.
- IgG – Antibodies with a higher binding strength and effect against the virus, which usually appear 8 to 10 days after infection.
Tests can show the presence of either or both types of antibodies. The peak for antibody production is usually between four and eight weeks after infection.
No one is sure yet exactly how long those antibodies will stay in the blood stream and be detectable. For some types of infections, like smallpox, antibodies are effectively with patients for life. For other coronaviruses, the levels appear to drop off over a period of years. In some cases, such as the common cold, it can be less than a year.
Generally, a more severe infection will result in the production of more antibodies, which will then also last longer.
What Are Antibody Tests Useful For?
Lateral flow tests use the same basic technology that is commonly used in pregnancy tests. That means it’s a well-understood mechanism, and there is plenty of capacity for producing and distributing the tests.
Because no processing is required, they are useful for rapid point-of-care testing that does not suffer from laboratory bottlenecks. They can be administered quickly and with minimal training.
On the other hand, they are not amenable to large batch testing, so they can be inefficient when testing large numbers of people, or large collections of blood, such as in blood banks.
Because antibody tests detect not the virus itself, but products of the body’s reaction to the virus, they are a lagging indicator of infections. They are not useful for reliably detecting a currently infected patient.
Although lateral flow and ELISA tests can also be used to detect antigens, which would determine current infection status, currently those in use for COVID-19 test only for antibodies.
For the same reason, however, they are more useful for determining whether or not a patient has had COVID-19 at some point in the not-too-distant past. This should also—although it has not been proven—mean that they continue to carry some degree of immunity to the disease and will not be reinfected.
The Complicated Question of Antibody-conferred Immunity to COVID-19
That understanding has been challenged by a number of cases where reinfection has been reported in China, Vietnam, and South Korea in patients who had already recovered from the disease. In the best-documented cases, in China, these were medical professionals, whose re-exposure would make them the most susceptible to reinfection.
The phenomenon does not seem to be widespread, since thousands of other healthcare staff are in a similar position. But it does challenge any simple assumption that a positive antibody test will equate to immunity.
On the other hand, there are also types of immunity that can be conferred without active antibodies present in the bloodstream. Although antibodies may not linger long, the B-cells that produce them, as the body’s immunity factory workers, can retain a memory, or blueprints, for generating them. That can lessen the time to generate them again in cases of reinfection, allowing the patient to fight off the disease that much faster, and possibly with fewer, or zero symptoms.
A similar effect can be achieved by a type of genetic immunity, which could explain segments of the population who are infected but remain asymptomatic. It’s unknown how broad this type of immunity might be, but antibody tests will not detect it.
How Accurate Are COVID-19 Antibody Tests?

Another major question surrounding current antibody tests for COVID-19 is their accuracy.
The limitations regarding the role of antibodies in immunity, and the time they take to develop and, possibly, ebb, are all factors in how accurate antibody tests are for detecting patients who have been infected.
But there are also test-specific accuracy issues regarding the sensitivity and specificity of the available assays. These factors can generate either false positives or false negatives, both of which can have a major impact depending on what the purpose of testing may be.
- Sensitivity – Highly sensitive tests have a higher true positive rate and are less likely to generate false negatives. In other words, a 100% sensitive test will always detect the presence of an antibody in blood where it actually exists.
- Specificity – A highly specific test has a higher true negative rate and is better at identifying when the antibody is not present, so it’s associated with a lower likelihood of false positives. A 100% specific test will never register the presence of the antibody if it is not actually present.
Most tests can’t hit 100 percent in both categories, and may not need to depending on the way they’re used. For example, if the test is being used to establish the extent of spread in the United States, false positives become a real problem for overestimating the possibility of developing herd immunity. That is exactly the issue that drew such intense criticism of a recent Stanford seroprevalence study that was found not to have sampled a large enough population, or corrected properly, for that sort of error.
The rate at which new tests are being release has not allowed rigorous independent verification. As of April 22nd, there were at least 11, both ELISA and lateral flow, some claiming 100% in both sensitivity and specificity. But they have all been rushed to market, some under emergency FDA authorization, and those claims may not hold up.
Eventually, antibody tests may form an essential part of the testing and tracing system that will have to be built to re-open society without sustaining more surges in hospitalizations and deaths.








